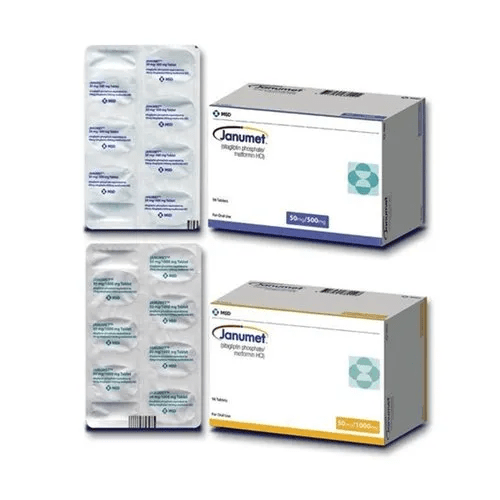
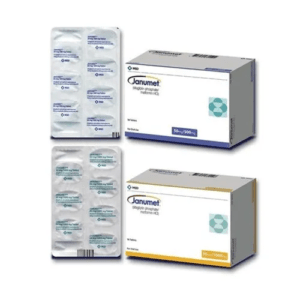
Sitagliptin Phosphate Metformin HCl
Product Details:
| Form | Tablet |
| Packaging Type | Box |
| Packaging Size | 56 Tablets |
| Composition | Sitagliptin Phosphate 50 mg, Metformin HCl 500 mg |
| Manufactured By | MSD |
| Brand | Janumet |
| Treatment | Control High Blood Sugar |
Description :
As initial therapy in type 2 DM when diet & exercise do not provide adequate glycemic control. Adjunct to diet & exercise to improve glycemic control in type 2 DM inadequately controlled on metformin or sitagliptin alone, or patients already receiving sitagliptin & metformin combination; may also be used in combination w/ a sulfonylurea, PPARγ agonist (eg, thiazolidinediones). Adjunct to diet & exercise to improve glycemic control in combination w/ insulin.
Dosage/Direction for Use:
- Individualized dosage while not exceeding max recommended daily dose of sitagliptin 100 mg. Initially sitagliptin 50 mg/metformin HCl 500 mg bid, may be titrated up to sitagliptin 50 mg/metformin HCl 1,000 mg bid. Patients inadequately controlled on metformin monotherapy Initially sitagliptin 50 mg bid metformin dose already being taken. Patients inadequately controlled on metformin monotherapy Usual starting dose: Sitagliptin 50 mg bid + metformin dose already being taken. Patients inadequately controlled on sitagliptin monotherapy Usual starting dose: Sitagliptin 50 mg/metformin HCl 500 mg bid, may be titrated up to sitagliptin 50 mg/metformin HCl 1,000 mg bid. Patients switching from co-administration of sitagliptin & metformin May be initiated at the dose of sitagliptin & metformin already being taken. Patients inadequately controlled on dual combination therapy w/ any 2 of these agents: Sitagliptin, metformin or a sulfonylurea, or a PPARγ agonist, or insulin Usual starting dose: Sitagliptin 50 mg bid. Determine dose of metformin based on glycemic control level & current metformin dose.
Administration:
- Should be taken with food.
Contraindications:
- Hypersensitivity. Severe renal impairment (eGFR <30 mL/min/1.73 m2), acute or chronic metabolic acidosis including diabetic ketoacidosis w/ or w/o coma. Intravascular administration of iodinated contrast materials (discontinue Janumet temporarily).
Special Precautions:
- Not for use in type 1 diabetes or diabetic ketoacidosis. History of pancreatitis. Assess renal function prior to initiation of therapy & at least annually thereafter. Hypoglycemia in combination w/ sulfonylurea or insulin. Discontinue if hypersensitivity reactions, & bullous pemphigoid occurs. Risk of lactic acidosis in patients w/ CHF requiring pharmacologic management, particularly those w/ unstable or acute CHF at risk of hypoperfusion & hypoxemia (promptly discontinue if these occurs); renal dysfunction; withhold use in the presence of any condition associated w/ hypoxemia, dehydration or sepsis; hepatic disease. Avoid excessive alcohol intake. Temporarily suspend therapy for any surgical procedure. Suspect lactic acidosis in any diabetic patient w/ metabolic acidosis lacking evidence of ketoacidosis (ketonuria & ketonemia). Elderly, debilitated or malnourished patients, & those w/ adrenal or pituitary insufficiency or alcohol intoxication are particularly susceptible to hypoglycemic effects, Concomitant use w/ drugs that may affect renal function or metformin disposition eg, cationic drugs. Temporarily discontinue intravascular contrast studies w/ iodinated materials at the time of or prior to the procedure, & withhold for 48 hr subsequent to the procedure & reinstitute after renal function has been reevaluated in patients w/ eGFR ≥30 to <60 mL/min/1.73 m
- history of hepatic impairment, alcoholism, or heart failure or patients who will be administered intra-arterial iodinated contrast. Promptly discontinue therapy if CV collapse, acute CHF, acute MI & other conditions characterized by hypoxemia have been associated w/ lactic acidosis & cause prerenal azotemia.
Related products
-
Anti Diabetic Medicine
Janumet XR CP Tablet
-
Anti Diabetic Medicine
Sitagliptin Phosphate Tablets IP
-
Anti Diabetic Medicine
Semaglutide Tablet 7 Mg
-
Anti Diabetic Medicine
Sitagliptin phosphate tablet 50 mg